
Answer:
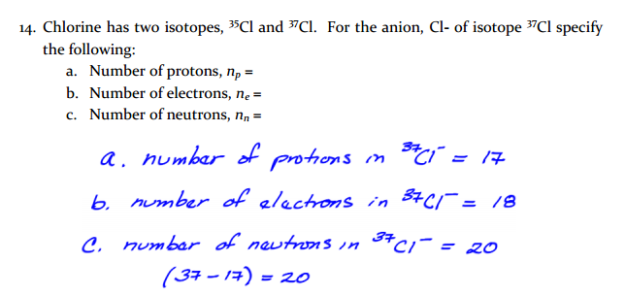
In general, there are two defining numbers for all elements and their isotopes: the mass number, and the atomic number. These are usually denoted as follows when an element is written:
AZ X
where A is the mass number, Z is the atomic number, and X is the symbol for the element in question (note that the A and Z would normally be aligned, not offset). The mass number, A, is the sum of the protons and neutrons in the element. The atomic number, Z, is the number of protons only. On the periodic table, elements are arranged by atomic number. In this case we see that Carbon is element 6, and so it had 6 protons. The number of neutrons alone can be easily calculated by subtracting the atomic number from the mass number, so that
N=A-Z
where N is the number of neutrons. The number of electrons in a given element, isotope, or ion, can be easily determined as well. In neutral isotopes, the number of negatively-charged electrons must balance the number of positively-charged protons.
Since the number of protons is given to us by the atomic number Z, this must also be the number of electrons. In ions, the plus or minus charge denoted tells us the overall charge of the species in question, and thus the lack or excess of electrons. A species with a +2 charge would have two less electrons than expected, while a -1 charge would mean one excess electron.

SOURCE: http://www.elementsdatabase.com/
SOURCE: http://www.ehow.com/how_8464533_number-protons-neutrons-isotope.html

SOURCE: http://www.esrl.noaa.gov/gmd/infodata/isotopes/chemistry.html
SOURCE: http://stuff.mit.edu:8001/afs/athena/course/3/3.091/www/probsetandquiz/PS1%20sol.pdf