To program with Python you can use Enthought IDLE program.
You can find it here: http://www.enthought.com/products/epdgetstart.php?platform=mac
To program with Python you can use Enthought IDLE program.
You can find it here: http://www.enthought.com/products/epdgetstart.php?platform=mac
Step1. Multiply density by volume and obtain grams of element
Step2. Obtain moles by dividing grams by atomic weight of element
Step3. Calculate number of atoms by multiplying moles by Avogrado number (6.022*10^23mol-1)
Example. How many atoms are in 18.0 cm3 of mercury (at room temperature)?
Density of mercury: 13.546 g/cm³
Atomic weight of mercury = 201g/mol
Density * Volume = 13.546 g/cm³ * 18cm3 = 243.828g
Moles = 244g/201g/mol = 1.214mol
Number of atoms = 1.214mol * 6.022*10^23mol-1 = 7.31*10^23
SOURCE: http://blip.tv/chemteam/mole-determine-number-of-atoms-when-given-density-and-volume-3418914
Atomic Weight Calculation
Calculating Atomic Weight of an Element with Isotopes
By Anne Marie Helmenstine, Ph.D., About.com Guide
See More About:
The atomic weight of an element depends on the abundance of its isotopes. If you know the mass of the isotopes and the fractional abundance of the isotopes, you can calculate the element’s atomic weight. The atomic weight is calculated by adding the mass of each isotope multiplied by its fractional abundance. For example, for an element with 2 isotopes:
atomic weight = massa x fracta + massb x fractb
If there were three isotopes, you would add a ‘c’ entry. If there were four isotopes, you’d add a ‘d’, etc.
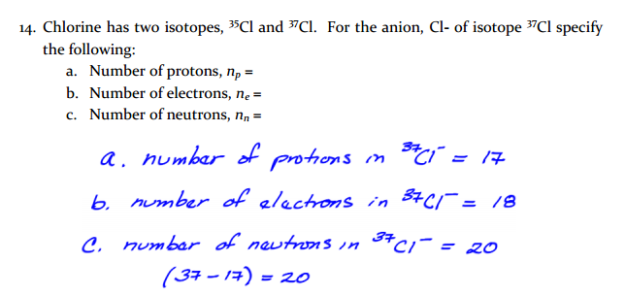
If chlorine has two naturally-occurring isotopes where:
Cl-35 mass is 34.968852 and fract is 0.7577
Cl-37 mass is 36.965303 and fract is 0.2423
atomic weight = massa x fracta + massb x fracb
atomic weight = 34.968852 x 0.7577 + 36.965303 x 0.2423
atomic weight = 26.496 amu + 8.9566 amu
atomic weight = 35.45 amu
Tips
The sum of the fractional abundance values must equal 1.
Be sure to use the mass or weight of each isotope and not its mass number.
Related Articles
SOURCE: http://chemistry.about.com/od/chemistryquickreview/a/atomicweight.htm

Answer:
In general, there are two defining numbers for all elements and their isotopes: the mass number, and the atomic number. These are usually denoted as follows when an element is written:
AZ X
where A is the mass number, Z is the atomic number, and X is the symbol for the element in question (note that the A and Z would normally be aligned, not offset). The mass number, A, is the sum of the protons and neutrons in the element. The atomic number, Z, is the number of protons only. On the periodic table, elements are arranged by atomic number. In this case we see that Carbon is element 6, and so it had 6 protons. The number of neutrons alone can be easily calculated by subtracting the atomic number from the mass number, so that
N=A-Z
where N is the number of neutrons. The number of electrons in a given element, isotope, or ion, can be easily determined as well. In neutral isotopes, the number of negatively-charged electrons must balance the number of positively-charged protons.
Since the number of protons is given to us by the atomic number Z, this must also be the number of electrons. In ions, the plus or minus charge denoted tells us the overall charge of the species in question, and thus the lack or excess of electrons. A species with a +2 charge would have two less electrons than expected, while a -1 charge would mean one excess electron.

SOURCE: http://www.elementsdatabase.com/
SOURCE: http://www.ehow.com/how_8464533_number-protons-neutrons-isotope.html

SOURCE: http://www.esrl.noaa.gov/gmd/infodata/isotopes/chemistry.html
SOURCE: http://stuff.mit.edu:8001/afs/athena/course/3/3.091/www/probsetandquiz/PS1%20sol.pdf
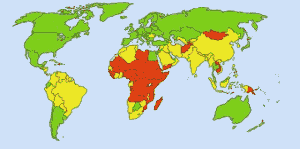
Safe Drinking Water: Image: http://commons.wikimedia.org/wiki/Special:Contributions/Ionut_Cojocaru. In green areas everyone can get safe water. In red areas, at least 25 percent of people can’t get treated water. Yellow areas are intermediate. Before the routine use of chlorine in U.S. water supplies, typhoid fever claimed 25 000 fatalities each year. Today 98% of public water supplies are treated with chlorine or a chlorine compound. Typhoid fever now claims about one victim a year, on average.

Discovery of Chlorine
Dr. Doug Stewart
Chlorine was first produced in 1774 by Carl Wilhelm Scheele in Sweden. Scheele collected the gas released by the reaction of pyrolusite [manganese dioxide] with spiritus salis – an alchemical term meaning spirit/breath of salt. [It was also known as muriatic acid and we now call it hydrochloric acid.] The new gas had, according to Scheele, “a very perceptible suffocating smell, which was most oppressive to the lungs… and gives the water a slightly acidic taste… the air in it acquires a yellow color…” (1)
Scheele also noted the high reactivity and the bleaching qualities of the new gas he had made: “…all metals were attacked… fixed alkali was converted into common salt… all vegetable flowers – red, blue, and yellow – became white in a short time; the same thing also happened with green plants… insects immediately died. (1)
Despite the accuracy of his observations, Scheele mistakenly thought the new gas was a dephlostiganated [* see below] form of muriatic acid.
The famous French chemist Antoine Lavoisier believed the new gas should be called oxymuriatic acid [an oxide of hydrochloric acid] based on the as yet undiscovered element murium. (2)
* The confusion about chlorine’s true identity was caused by the phlogiston theory; phlogiston had been accepted by chemists for most of the 1700s – until Lavoisier himself debunked it. Phlogiston was a ‘substance’ used to explain the then inexplicable. Conveniently, it had negative weight when it needed to, and ‘explained’ reactions such as rusting and burning.
Lavoisier was the architect of phlogiston’s downfall, showing that the chemistry of oxygen was a better explanation in chemical reactions than phlogiston was. (3)
By 1810 the scientific consensus was that the element we now call chlorine was actually a compound that contained oxygen. English chemist Sir Humphry Davy found that the consensus was wrong; he could not get the new yellow-green gas to react with a charcoal electrode, which made him believe it may not contain oxygen. In reactions with phosphorus and ammonia, he demonstrated the new gas did not contain oxygen. He used a huge, 2000 plate voltaic pile [battery] to see whether he could extract oxygen from the gas’s phosphorus and sulfur compounds, but again he found no oxygen. (1a)
In 1811, Davy concluded the new gas was in fact a new element. (1b) He named it chlorine, from the Greek word ‘chloros,’ meaning pale green or yellow-green.
Interesting Facts about Chlorine
The first chain reaction discovered was not a nuclear reaction; it was a chemical chain reaction. It was discovered in 1913 by Max Bodenstein, who saw a mixture of chlorine and hydrogen gases explode when triggered by light. The chain reaction mechanism was fully explained in 1918 by Walther Nernst.
Earth’s oceans contain a large amount of chlorine. If this chlorine were released as a gas, its weight would be 5x greater than Earth’s total current atmosphere. (Our oceans contain about 2.6 x 1016 metric tons of chlorine, mostly as sodium chloride.)
Chlorine is not only abundant in our oceans; it is the sixth most abundant element in Earth’s crust.
Exposure to small amounts of chlorine, even for a short time, can be deadly. Fatalities are expected at 1 part in a thousand chlorine in air (or sometimes at even lower concentrations). (5)
Chlorine is heavier than air. When released, it forms a poisonous blanket that drifts along with the wind. Chlorine was used as a chemical weapon in World War I, first in 1915 by the German army and then by the Western Allies. It was not as ‘effective’ as had been hoped, because chlorine is easily detected by its strong smell. It is also water soluble, and so soldiers could protect themselves from the worst of its effects by breathing through damp cloths.
Uses of Chlorine
Chlorine is used for producing safe drinking water.
Chlorinated compounds are used mostly for sanitation, pulp bleaching, disinfectants, and textile processing.
Chlorine is also used for the manufacture of chlorates and it is important in organic chemistry, forming compounds such as chloroform, carbon tetrachloride, polyvinyl chloride, and synthetic rubber.
Other uses of chlorine compounds include dyestuffs, petroleum products, medicines, antiseptics, insecticides, foodstuffs, solvents, paints and plastics.
Abundance and Isotopes
Abundance earth’s crust: 145 parts per million by weight, 85 parts per million by moles
Abundance solar system: 8 parts per million by weight, 0.3 parts per million by moles
Cost, pure: $0.15 per 100g
Cost, bulk: $ per 100g
Source: Chlorine gas is produced commercially by the electrolysis of sodium chloride (NaCl) from seawater or brine from salt mines.
Isotopes: Chlorine has 16 isotopes whose half-lives are known, with mass numbers 31 to 46. Naturally occurring chlorine is a mixture of its two stable isotopes 35Cl and 37Cl with natural abundances of 75.8% and 24.3% respectively.
References
1. Henry M. Leicester, Herbert S. Klickstein, A Source Book in Chemistry, 1400-1900., (1969) p111. Harvard University Press.
1a. Henry M. Leicester, Herbert S. Klickstein, A Source Book in Chemistry, 1400-1900., (1969) p241. Harvard University Press.
1b. Henry M. Leicester, Herbert S. Klickstein, A Source Book in Chemistry, 1400-1900., (1969) p257. Harvard University Press.
2. J.W. Mellor, A Comprehensive Treatise on Inorganic and Theoretical Chemistry., 1922, vol 2, Longmans, Green and Co., p21.
3. Antoine Lavoisier, Memoires de l’ Academie royale des sciences 1783., 1786, p505-538.
4. The Chlorine Tree.
5. OSHA, Occupational Safety and Health Guideline for Chlorine.
SOURCE: http://www.chemicool.com/elements/chlorine.html
“Chlorine.” Chemicool Periodic Table. Chemicool.com. 16 Oct. 2012. Web. 2/20/2013